
Precio FOB
Obtener el precio más reciente0.1 ~ 2 USD / Piece
|100 Box Minimum Order
País:
China
N º de Modelo:
JG001
Precio FOB:
0.1 ~ 2 USD / Piece Obtener el precio más reciente
Lugar de origen:
CHINA
Precio de pedido mínimo:
0.1 per Piece
Cantidad de pedido mínimo:
100 Box
Detalle de embalaje:
CARTON
El tiempo de entrega:
7 DAYS
Capacidad de suministro:
1000000 Box per Week
Tipo de pago:
T/T, PayPal
Grupo de productos :
China
Persona de contacto Ms. jazmin
3rd.street, No.28, Hangzhou, Zhejiang
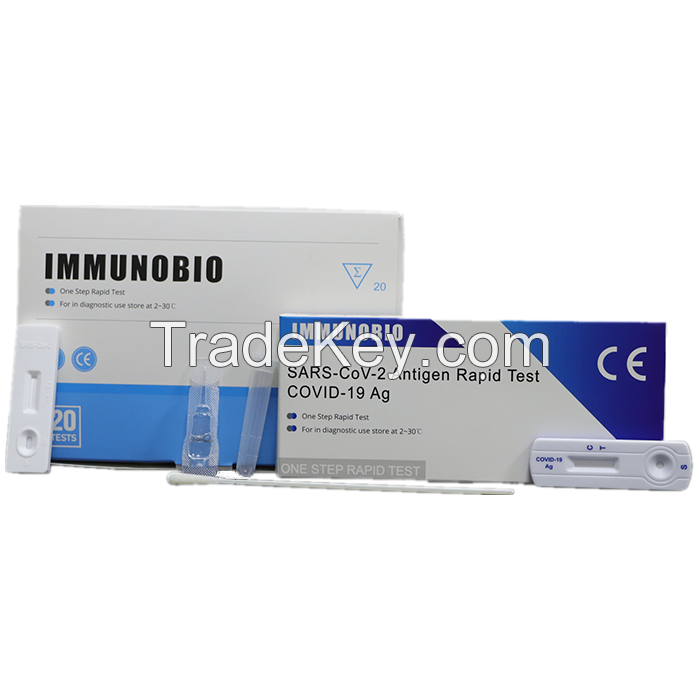
INTENDED USE
The SARS-CoV*2 Antigen Rapid Test (COVID**9
Ag) is a rapid chromatographic immunoassay for the qualitative
detection of novel coronavirus SARS-CoV*2 in human throat
and the front
of nasal secretions.
SPECIMEN COLLECTION AND
PREPARATION
The SARS-CoV*2 Antigen Rapid Test
(COVID- *9 Ag) can be performed using throat
secretions and nasal secretions.
· Throat Secretions: Insert the sterile swab
into the throat. Gently scrape the secretions around the wall of
pharynx.
· The front of
nasal
Secretions: The sterile
swab was inserted 2.5cm into the nasal
cavity.Gently rotate the swab against the wall of the
turbinate several times to wet the swab as much as
possible.
· Collect 0.5ml of
assay buffer and
place into a specimen collection
tube. Insert the swab into the tube and squeeze
the flexible tube to extrude the specimen from the head of the
swab. Make the specimen resolved in the assay buffer sufficiently.
Add the crystal tip onto the specimen collection tube. If saliva
specimen, suck the saliva from the container and place 5 drops
(approx.**0ul) of the saliva into the sample collection
tube.
The assay should be performed immediately in 2
hours after the specimen preparation. If the assay could not be
carried immediately, the prepared specimen should be kept no more
than *4 hours at **8°C or 7 days at **0°C.
Bring specimens to room temperature prior to
testing. Frozen specimens must be completely thawed and mixed well
prior to testing. Specimens should not be frozen and thawed
repeatedly for more than two times.
If specimens are to be shipped, they should be
packed in compliance with federal regulations covering the
transportation of etiologic agents.
TEST
PROCEDURE
Allow the test
device, specimen, buffer, and/or controls to equilibrate to room
temperature (****0°C) prior to testing.
1. Bring the pouch to room temperature before
opening. Remove the test device from the sealed pouch and use it as
soon as possible.
2. Place the test device on a clean and
horizontal surface. Reverse the specimen collection tube, extrude 3
drops of the prepared specimen into the specimen well (S) of the
test cassette and start the timer.
See illustration
below.
3. Wait for the
colored line(s) to appear. Read results at *0 minutes. Do not
interpret the result after *5 minutes.
INTERPRETATION OF
RESULTS
- Positive
(+): Two colored lines appear. One colored line
should always appear in the control line region (C) and another
line should be in the T line region.
*NOTE: The
intensity of the color in the test line regions may vary depending
on the concentration of SARS-CoV*2 present in the specimen.
Therefore, any shade of color in the test line region should be
considered positive and recorded as
such.
- Negative
(-): One colored line appears in the control line
region (C). No line appears in the T line
region.
Invalid:
Control line fails to appear. Insufficient specimen volume or
incorrect procedural techniques are the most likely reasons for
control line failure. Review the procedure and repeat the test with
a new test. If the problem persists, discontinue using the test kit
immediately and contact your local
distributor.
| País: | China |
| N º de Modelo: | JG001 |
| Precio FOB: | 0.1 ~ 2 / Piece Obtener el precio más reciente |
| Lugar de origen: | CHINA |
| Precio de pedido mínimo: | 0.1 per Piece |
| Cantidad de pedido mínimo: | 100 Box |
| Detalle de embalaje: | CARTON |
| El tiempo de entrega: | 7 DAYS |
| Capacidad de suministro: | 1000000 Box per Week |
| Tipo de pago: | T/T, PayPal |
| Grupo de productos : | Human Disease Rapid Test |