
Precio FOB
Obtener el precio más reciente0.1 ~ 2 USD / Piece
|100 Box Minimum Order
País:
China
N º de Modelo:
HMKP054G
Precio FOB:
0.1 ~ 2 USD / Piece Obtener el precio más reciente
Lugar de origen:
China
Precio de pedido mínimo:
0.1 per Piece
Cantidad de pedido mínimo:
100 Box
Detalle de embalaje:
carton
El tiempo de entrega:
7 days
Capacidad de suministro:
1000000 Box per Week
Tipo de pago:
PayPal, T/T
Grupo de productos :
China
Persona de contacto Ms. jazmin
3rd.street, No.28, Hangzhou, Zhejiang
INTENDED
USE
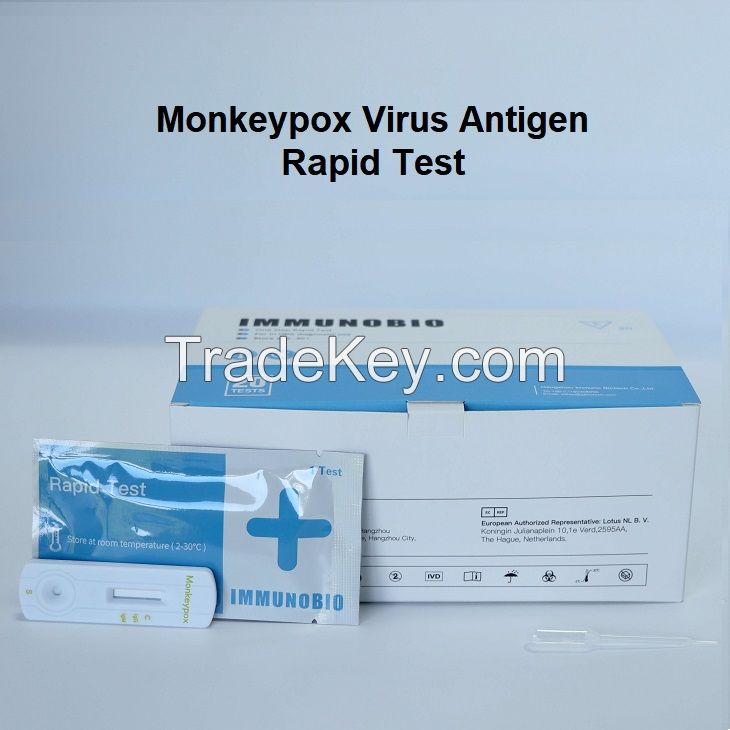
TheMonkeypox
Virus Antigen Rapid
Test is a rapid chromatographic immunoassay
for the qualitative detection
ofMonkeypox antigen
in humanskin
lesionswab,
ormucous
membraneswab
specimen inindividuals
who are suspected ofmonkeypox-like symptoms or recent contact
with monkeypox infected
patients.
SPECIMEN COLLECTION AND
PREPARATION
TheMonkeypox
Virus Antigen Rapid
Test can be
performed
usingskin
lesion ormucous
membrane swab.
Skin
lesion Swab: Place the
sterile swab onto
the skin lesion site, especially the part with vesicles. Gently
scrape the lesion to
collect the fluid for 5~8 times with the
swab.
Mucous
membrane Swab:Placethe head
of sterile swab into
thedeepthroat, or the
nasal cavities, or onto the eye
conjunctiva. Gently scrape
themucous
membrane with the
swab for 5~8 times.
Take out of an
assay buffer tube, and tear off
theseal of
the tube. Insert the swab into the tube and squeeze the flexible
tube for
8~*0 times to extrude the specimen from the head
of the swab. Make the specimen resolved in the assay buffer
sufficiently. Add the tip onto the assay
buffer tube.
The assay should be performed immediately in
2 hours after the specimen preparation. If the assay could not be
carried immediately, the prepared specimen should be kept no more
than *4 hours at **8°C or 7 days at **0°C.
Bring specimens to room temperature prior to
testing. Frozen specimens must be completely thawed and mixed well
prior to testing. Specimens should not be frozen and thawed
repeatedly for more than two times.
If specimens are to be shipped, they should
be packed in compliance with federal regulations covering the
transportation of etiologic agents.
TEST
PROCEDURE
Allow
the rapid
test, specimen, buffer, and/or controls to
equilibrate to room temperature (****0°C) prior to
testing.
1. Bring the pouch to room temperature before
opening. Remove therapid test
cassettefrom the sealed pouch and use it as soon as
possible.
2. Place the test device on a clean and
horizontal surface. Reverse the specimen collection tube, extrude 3
drops of the prepared specimen into the specimen well (S) of the
test cassette and start the timer.
3. Wait for the colored line(s) to appear. Read
results at *0 minutes. Do not interpret the result after *5
minutes.
INTERPRETATION OF
RESULTS
POSITIVE: Two colored
bands appear on the membrane. One
band appears in the control region (C) and another band appears in
the test region (T).
NEGATIVE: Only one colored band appears in
the control region (C).No colored
band appears in the test region (T).
INVALID: Control
band fails to
appear.Results
from any test which has not produced a control band at the
specified read time must be discarded. Please review the procedure
and repeat with a new test. If the problem persists, discontinue
using the kit immediately and contact your local
distributor.
NOTE:
1. The
intensity of color in the test region (T) may vary depending on the
concentration of analytes present in the specimen. Therefore, any
shade of color in the test region should be considered positive.
Note that this is a qualitative test only, and cannot determine the
concentration of analytes in the specimen.
2. Insufficient specimen volume, incorrect
operating procedure or expired tests are the most likely reasons
for control band failure.
| País: | China |
| N º de Modelo: | HMKP054G |
| Precio FOB: | 0.1 ~ 2 / Piece Obtener el precio más reciente |
| Lugar de origen: | China |
| Precio de pedido mínimo: | 0.1 per Piece |
| Cantidad de pedido mínimo: | 100 Box |
| Detalle de embalaje: | carton |
| El tiempo de entrega: | 7 days |
| Capacidad de suministro: | 1000000 Box per Week |
| Tipo de pago: | PayPal, T/T |
| Grupo de productos : | Human Disease Rapid Test |