China China
Persona de contacto Greta
Dirección China
Contactar ahora Califique a esta empresa Informe sobre Fraude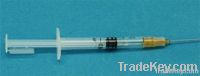 0.3ml Retractable safety syringe auto-disable syringe AD syringe safe
0.3ml Retractable safety syringe auto-disable syringe AD syringe safe
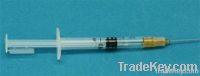 0.5ml Retractable safety syringe auto-disable syringe AD syringe safe
0.5ml Retractable safety syringe auto-disable syringe AD syringe safe
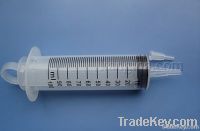 100ml feeding syringe
100ml feeding syringe
 100ml luer lock syringe
100ml luer lock syringe
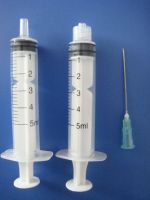 10ml disposable syringe
10ml disposable syringe
 14-31G Hypodermic needle
14-31G Hypodermic needle
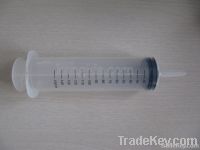 150ml feeding syringe
150ml feeding syringe
 1ml disposable syringe
1ml disposable syringe
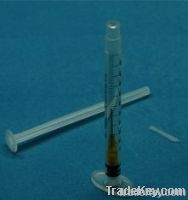 1ml Retractable Safety Single Use Only Syringe
1ml Retractable Safety Single Use Only Syringe
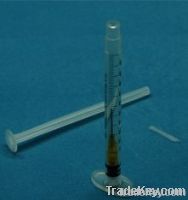 1ml Retractable safety syringe auto-disable syringe AD syringe safe
1ml Retractable safety syringe auto-disable syringe AD syringe safe
UNIONMED main founder Mr. Zhang Wenguo has been involved in the R&D and production of the Disposable High Pressure Syringe during the last two decades. As the first maker of the enterprise standards regarding this product, Mr. Zhang Wenguo is also one of the main makers of the industry standards in China. So he is known as the first person in this industry, he is experienced in technical research and quality control.
UNIONMED products are CFDA registered, CE certified and FDA approved. We are now selling to more than *0 countries and regions worldwide. We live with our quality, we make our future with innovation, and we realize our cooperation with mutual benefits. Connect with us, for a BETTER U, BETTER US.
Contactar ahora| Tipo de Comercio | Manufacturing |
| Sitio Web | https://www.unionmedsyringe.com/ |
| Año de Constitución | 2015 |
| Number of Employees | 1000-2000 |
| Principales Mercados | Worldwide |
| Company Products / Services | ct scan contrast media injector syringe, angiography syringe, connector tube |
| Localización de la fábrica | Shenzhen |
| Ubicaciones de fábrica | 3000 sqm to 5000 sqm |
| No. de líneas de producción | 3 |
| Volumen de compra anual total | N/A |
| Nº de personal de I + D | 6 - 10 people |
| Control de calidad | N/A |
| Certificado | ISO, CE, FDA 510K |
| Fabricación por contrato | OEM Service Offered ,Buyer Label Offered |
| Capital registrado | N/A |
| Tipo de Propiedad | N/A |
| Representante Legal / Director Ejecutivo | N/A |
| Porcentaje de exportación | N/A |
| Volumen total de ventas anuales | N/A |
| No. de personal de control de calidad | N/A |
| Persona de contacto | Greta |
| Empresa | Union Medical Shenzhen Co., Ltd. |
| teléfonos | ******** |
| Mobile | ******** |
| Fax | ******** |