FOB Price
Obtener el precio más reciente|
Minimum Order
Place of Origin:
-
Price for Minimum Order:
-
Minimum Order Quantity:
-
Packaging Detail:
-
Delivery Time:
-
Supplying Ability:
-
Payment Type:
-
Persona de contacto Sangam
Hodal, Haryana
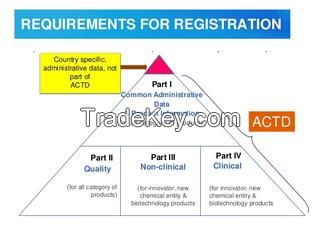
ACTD Format Dossier is also Described as ASEAN CTD Dossier, ASEAN Common Technical Dossier (ACTD) provides a common format for the preparation of well-structured Common Technical Dossier applications for submission in ASEAN regulatory authorities for the registration of pharmaceuticals for human use. ACTD format significantly reduce the time and resources needed to compile applications for registration. Regulatory reviews and communication with the applicant is facilitated by a standard document of common elements.
This guideline merely demonstrates an
appropriate write-up format for acquired data. However,applicants
can modify, if needed, to provide the best possible presentation of
the technical information, in order to facilitate the understanding
and evaluation of the results upon pharmaceutical
registration.
Dossier writing and compilation as per
ACTD Format.
Asian Common Technical Documents
consists of following parts.
Part I Administrative Data and Product Information
Part II Quality Documents
Part III Non Clinical Documents
Part IV Clinical Documents.
ACTD Format is Asian harmonization for Common technical Documents
used in
Asian Countries like Vietnam, Thailand, Singapore, and Malaysia, Philippines
& in Member States.