





Precio FOB
Obtener el precio más reciente( Negotiable )
|50000 Piece Minimum Order
País:
Others1
N º de Modelo:
11901
Precio FOB:
( Negotiable ) Obtener el precio más reciente
Lugar de origen:
Others1
Precio de pedido mínimo:
-
Cantidad de pedido mínimo:
50000 Piece
Detalle de embalaje:
as per attach photo or brochure
El tiempo de entrega:
As per cutomer requirements
Capacidad de suministro:
300000 Piece per Day
Tipo de pago:
T/T
Grupo de productos :
Persona de contacto Mr. Sam
Room 302C, No.718, Blv San Yuan Li, Bai Yuan District,, Changping, Guangdong
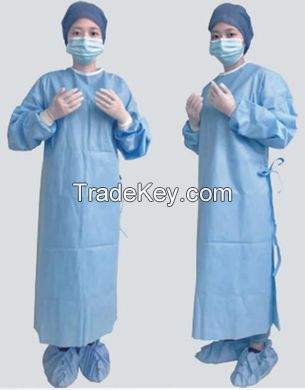
Product
Name: Sterile
Surgical Suit
Model No.:According
to Customer Specification
Certificate:
510K No.:
K192290
TUV CE Based on EN
13795-1:2019 (CE
No.: G2S
073966 0008 Rev. 01)
TUV Level 4 Test Report from TUV, Based on
AAMI PB70:2012
TUV Level 3 Test Report Based on AAMI
PB70:2012
TUV Level 2 Test Report Based on AAMI
PB70:2012
TUV
ISO 13485
We herewith declare that the
above mentioned products meet the transposition into national law,
the provisions of the following EC Council directives and
Standards. All supporting documentations are retained under the
premises of the manufacturer.
UMDNS
Code:11901
Classification (MDD Annex
IX): I
Sterile, Rule 1
Conformity Assessment
Route:Annex
V section 3
General applicable
directives:
Medical Device Directive: COUNCIL
DIRECTIVE 93/42/EEC
Standard
Applied:
EN ISO 15223-1:2016
EN1041:2008 EN ISO
13485:2016
EN ISO 14971:2012
EN 13795:2011
Notified
Body:TV
SD Products Service GmbH, Ridlestr. 65, 80339, Munchen,
Germany
Identification
Number:0123
(EC)
Certificate(S):G2S
073966 0008 Rev.01
Expire Date of the
Certificate:2024-05-26
Start of CE
Marking:2010-11-26
Place,Date of
Issue:Xuchang,
2019-10-15
*Ergonomic fit, enables freedom
of movement
*Reinforced
*Breathable and comfortable,
suitable for most procedures
*Specially treated to repel
low-tension surface fluids
Manufacturing
Center:10
Sterilization Center:
2
Employee:
4000
RD
Center:2
Market: 74
Countries
| País: | Others1 |
| N º de Modelo: | 11901 |
| Precio FOB: | ( Negotiable ) Obtener el precio más reciente |
| Lugar de origen: | Others1 |
| Precio de pedido mínimo: | - |
| Cantidad de pedido mínimo: | 50000 Piece |
| Detalle de embalaje: | as per attach photo or brochure |
| El tiempo de entrega: | As per cutomer requirements |
| Capacidad de suministro: | 300000 Piece per Day |
| Tipo de pago: | T/T |
| Grupo de productos : | surgical suit |